The article “The Invasion Equation. Will a tumor spread? That may depend as much on your body as on your cancer” by Siddhartha Mukherjee in the New Yorker nicely explains metastasis or how tumour cells spread in the body.
Author: Neuroblast
What lessons have been learnt?
 Today is the final day of the Third International Cancer lmmunotherapy Conference. The meeting was run at the Rheingoldhalle Congress Center in Mainz/Frankfurt, Germany from September 6-9, 2017. More than 500 people attended this meeting.
Today is the final day of the Third International Cancer lmmunotherapy Conference. The meeting was run at the Rheingoldhalle Congress Center in Mainz/Frankfurt, Germany from September 6-9, 2017. More than 500 people attended this meeting.
The focus of the scientific program was on “Translating Science into Survival”. Talks covered the challenging areas in cancer immunology and immunotherapy. The full list of topics can be found in the meeting program.
At the moment cancer immunology and immunotherapy is a hot topic in the next generation of anti-cancer therapies. Lots of attention is given to checkpoint immunodrugs as it was proven by the prevalence of talks on this subject in the program. Indeed, this drug has great potential, but at the same time, it is not universal. About 50% of patients do not benefit from it.
What lessons have been learned from the talks:
- Checkpoint immunotherapies are the main stream
- Not all cancer patients would respond to immunodrug
- Genetic landscape of a tumour and/or the patient may contribute to this, thus making beneficial to check genetics for this type of treatment
- Immunodrugs work better in combination with conventional therapies such as chemotherapy.
- The immune system can be tuned by a drug, but it will switch on compensatory mechanisms to balance the intervention.
- Lots have to be studied further
![]()
Father of Chemotherapy and Cancer Immunology
 I was giving a talk at Georg-Speyer-Haus Institute for Tumour Biology and Experimental Therapy yesterday. The aim of my visit was to establish collaboration with Prof Daniela Krause, who is the expert in bone marrow microenvironment and targeted therapies. She took me to the Institute museum that keeps the history of this place and phenomenal researchers used to work there.
I was giving a talk at Georg-Speyer-Haus Institute for Tumour Biology and Experimental Therapy yesterday. The aim of my visit was to establish collaboration with Prof Daniela Krause, who is the expert in bone marrow microenvironment and targeted therapies. She took me to the Institute museum that keeps the history of this place and phenomenal researchers used to work there.
This research institute was established in 1904 to support work of Paul Ehrlich, its first director and funded by the private foundation “Chemotherapeutisches Forschungsinstitut Georg-Speyer-Haus”. Paul Erlich is the Father of the chemotherapy concept originally developed to treat diseases of bacterial origin. He reasoned that there should be a chemical compound that can specifically target bacteria and stop its growth. He developed Salvarsan, the most effective drug for treatment of syphilis until penicillin came onto the market.
Paul Erlich is also known for his contribution to cancer research. He and his colleagues actively experimented on how tumour originates and spread. They also tried to understand how immune system can beat cancer applying vaccination concepts.

Paul Erlich and Ilya Mechnikov were jointly awarded The Nobel Prize in Physiology or Medicine for his “work on immunity” in 1908.

Tumour immunology and immunotherapy for neuroblastoma
The main challenge in treating high-risk neuroblastoma is to stop or control tumour spread and development of resistance to multiple chemotherapeutic drugs. Immunotherapy is one of the recent advances in our understanding how our immune system handles body invaders such as virosis, bacteria and now tumour cells. Immunotherapy holds great promise as a treatment option for neuroblastoma as well as for many adult cancers owing to the specificity of immune effector cells targeted to a tumour. Another advantage is a potential reduction in the systemic side effects observed with other forms of treatment.
This video ‘Tumour immunology and immunotherapy’ will give a brief overview of the basic concepts.
Immunotherapeutic approaches for neuroblastoma include the use of chimeric antigen receptor (CAR) T cells against both L1-CAM and ganglioside 2 (GD2) cell surface antigens to promote host antitumor response. Anti-GD2 antibodies bind GD2 and cause cell death by activating both complement-dependent cytotoxicity (CDC) and AB-dependent cellular cytotoxicity (ADCC) from natural-killer cells.
Treatment of High-Risk Neuroblastoma
Children with high-risk neuroblastoma is the most challenging group to treat. Current treatment strategy for this group consists of 3 treatment blocks:
- induction: chemotherapy and primary tumour resection;
- consolidation: high-dose chemotherapy with autologous stem-cell rescue and external-beam radiotherapy [XRT];
- post-consolidation: anti–ganglioside 2 immunotherapy with cytokines and cis-retinoic acid.
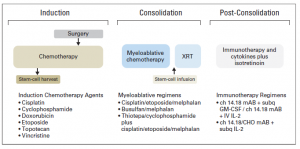
Pinto NR, Applebaum MA, Volchenboum SL, Matthay KK, London WB, Ambros PF, Nakagawara A, Berthold F, Schleiermacher G, Park JR, Valteau-Couanet D, Pearson AD, Cohn SL. Advances in Risk Classification and Treatment Strategies for Neuroblastoma.J Clin Oncol. 2015 Sep 20;33(27):3008-17.
What is the risk group classification system?
To be able to guide the treatment of neuroblastoma patients, doctors have developed a number of classification systems. Although sharing common features, they slightly vary by medical center, country and continents making direct comparisons of treatment results difficult. Doctors and scientists are trying to consolidate all systems in one in order to evaluate treatments in the past, currently ongoing and in the future.
Scientists have suggested a newer risk group classification system, the International Neuroblastoma Risk Group (INRG) classification that would incorporate the best knowledge gained and recent advancements in the disease imaging and neuroblastoma molecular diagnostics. This system is based on imaging criteria using the image-defined risk factors (IDRFs) and the prognostic factors such as:
- The child’s age
- Tumour histology (the tumour appearance under the microscope)
- The presence or absence of MYCN gene amplification
- Certain changes in chromosome 11 (known as an 11q aberration)
- DNA ploidy (the total number of chromosomes in the tumour cells)
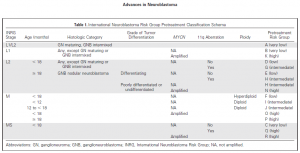
Using these factors the INRG classification put children into 16 different pre-treatment groups (lettered A through R). Each of these pretreatment groups is within 1 of 4 overall risk groups:
- Very low risk (A, B, C)
- Low risk (D, E, F)
- Intermediate risk (G, H, I, J)
- High risk (K, N, O, P, Q, R)
This system has not yet become common across all medical centers, but it is being researched in new treatment protocols.
Doctors and scientists are planning to improve the INRG classification system by incorporating other molecular diagnostics data such as profiles of the neuroblastoma genome (DNA), transcriptome (RNA), and epigenome* in order to make precise prognostication even better.
References:
Pinto NR, Applebaum MA, Volchenboum SL, Matthay KK, London WB, Ambros PF, Nakagawara A, Berthold F, Schleiermacher G, Park JR, Valteau-Couanet D, Pearson AD, Cohn SL. Advances in Risk Classification and Treatment Strategies for Neuroblastoma.J Clin Oncol. 2015 Sep 20;33(27):3008-17.
What is neuroblastoma?
Neuroblastoma is a childhood cancer. The word neuroblastoma consists of two words neuro and blastoma.The term neuro refers to nerves, blastoma – to a cancer of immature cells.
It starts in some types of nerve cells during embryo development.transforming immature nerve cells into cancerous cells. This type of cancer occurs most often in infants and young children mostly under the age of 5 years old.
Neuroblastomas behave very differently:
- Cells can grow and spread quickly,
- Cells can grow slowly
- Cells can die for no reason, so a tumour goes away on its own.
- Cells can mature on its own into normal ganglion cells and stop dividing,
The types of treatment used for neuroblastoma can include:
- Surgery
- Chemotherapy (the video explains how chemotherapy works)
- Radiation therapy
- High-dose chemotherapy/radiation therapy and stem cell transplant
- Retinoid therapy
- Immunotherapy
Children who survive have a high chance of developing long term side effects as a result of the treatment that saved their lives
More details about neuroblastoma can be found here:
What is cancer?
Cancer is an umbrella term that covers a group of diseases sharing the common features but diseases vary by site of origin, tissue type, race, sex, and age. One of the main features is an uncontrollable growth of cells. These cells are capable of spreading to other parts of the body. This process is also known as invasion and metastasis.
Though cancer in kids is not the same as in adults, childhood cancer cells behave in the same way. They grow uncontrollably and can travel to new destinations in the body.
This video ‘Cancer: from a healthy cell to a cancer cell’ nicely explains this transformation.
This video ‘How does cancer spread through the body?’ gives a perspective on the ways cancer cells travel in the body.
September is Childhood Cancer Awareness Month
September is Childhood Cancer Awareness Month!
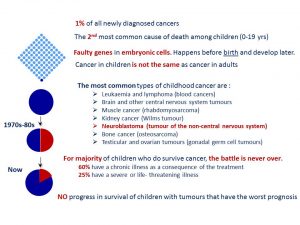
Facts about childhood cancer
Please watch this video created by St. Baldrick’s Foundation | The Childhood Cancer Ripple Effect
References:
Gatta G, Botta L, Rossi S, Aareleid T, Bielska-Lasota M, Clavel J, et al. Childhood cancer survival in Europe 1999-2007: Results of EUROCARE-5-a population-based study. Lancet Oncol. 2014;15(1):35–47.
Ward E, Desantis C, Robbins A, Kohler B, Jemal A. Childhood and Adolescent Cancer Statistics, 2014. Ca Cancer J Clin. 2014;64(2):83–103.
Dolgin MJ, Jay SM. Childhood cancer. 1989;327–40.
Miller RW, Young Jr. JL, Novakovic B. Childhood cancer. Cancer [Internet]. 1995;75(1 Suppl):395–405.
Raab CP, Gartner JC. Diagnosis of Childhood Cancer. Primary Care – Clinics in Office Practice. 2009. p. 671–84.
Howlader N, Noone A, Krapcho M, Garshell J, Miller D, Altekruse S, et al. SEER Cancer Statistics Review, 1975-2011 [Internet]. National Cancer Institute. 2014.
Ries L a. G, Smith M a., Gurney JG, Linet M, Tamra T, Young JL, et al. Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995. NIH Pub No 99-4649. 1999;179 pp
Warren KE. Diffuse intrinsic pontine glioma: poised for progress. Front Oncol [Internet]. 2012;2(December):205.
Lackner H, Benesch M, Schagerl S, Kerbl R, Schwinger W, Urban C. Prospective evaluation of late effects after childhood cancer therapy with a follow-up over 9 years. Eur J Pediatr. 2000;159(10):750–8.
SIOPEN Meeting 2017
The dates for the SIOPEN AGM and Neuroblastoma Research Symposium were announced. This meeting will be hosted by the German Society for Paediatric Oncology and Haematology and will take place @ Langenbeck Virchow Haus, Berlin, Germany on October 25-27, 2017.
Mission
To increase the understanding of neuroblastoma pathogenesis,
progression and treatment failure and to improve survival
and quality of life for children with neuroblastoma.
Main Objectives
- To consolidate a platform for global collaboration
- To establish networks of multidisciplinary caregivers
- To develop new trial protocols
- To develop standards for radiotherapy and surgery
- To develop SOPs for biomaterial collection, handling and storage
- To develop SOPs for application of major research technologies
- To identify leaders for specific topics
Scientific Topics/Plenary Sessions
- Molecular Risk Stratification
- Liquid Biopsies
- Tumor Heterogeneity+Tumor Microenvironment
- New Preclinical Models: PDX, GEMM, zebrafish
- New Immunotherapy Approaches
- New Drug Targets/Early Clinical Trials
- Neuroblastoma Pathogenesis/Genetics
- Targeting MYC
- Targeting ALK
- Targeting RAS/MAPK
- SIOPEN HR-NBL2 Clinical Trial Strategy
- Update ongoing SIOPEN trials: HR-NBL-1, LINES, VERITAS, OMS
- Update SIOPEN Bioportal
- Concept Biology+Relapse Umbrella Trial

