Hi, it’s Alysia, here to give you some insight on exosomes’ impact on tumor metastatic niche formation! I found an interesting paper related to my work that I want to share with you: Tumour-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming.
Exosomes have been a groundbreaking field of research due to their hypothesized ability to create a pre-metastatic niche through non-cancerous cell manipulation. They achieve this by relaying oncogenes and proteins from cancer cells in their molecular “cargo”. In this study, Morrissey et al. examined the role of tumor-derived exosomes (TDEs) in increasing programmed death ligand-1 (PDL-1) expression in macrophages. In pre-metastatic tumour microenvironments, there is an increase in inflammation and an immunosuppressive response, thus promoting tumorigenesis. PDL-1 is increased by TDEs manipulating the signalling of toll-like receptor 2 (TLR2) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), which increases lactate production and glucose uptake in an event called the “Warburg Effect”, which is common in tumour microenvironments. TDEs taken from lung cancer samples were injected into mice, and PDL-1 expression levels of macrophages were higher than in healthy lung samples.
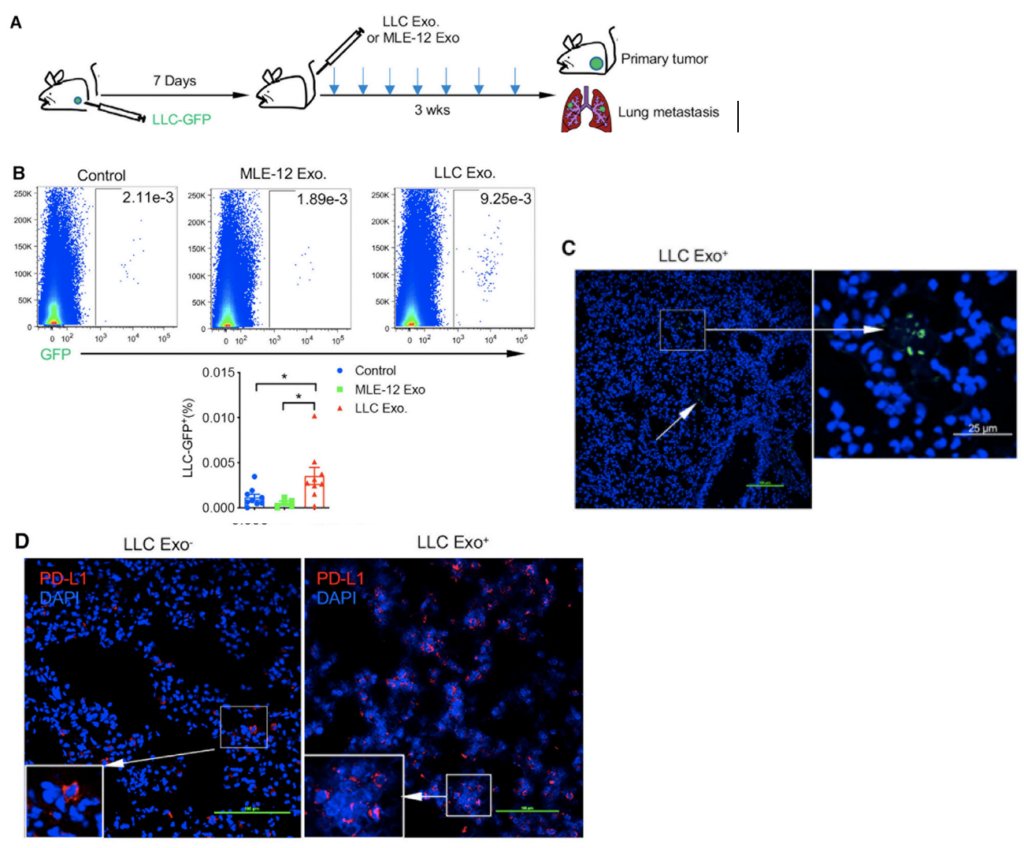
To accomplish this, Lewis lung carcinoma tumour cells (derived from mice) labelled with a green fluorescent protein (LLC-GFP+) were injected into mice with tumours; seven days later, they were injected with either non-GFP LLC exosomes or control mouse lung epithelial cell exosomes (MLE-12). The mice injected with non-GFP LLC exosomes had no metastatic difference compared to the control mice. However, the mice injected with LLC-GFP+ exosomes had an increase in micro-metastases, which was confirmed using fluorescent microscopy to observe LLC-GFP+ occurrence. With confocal microscopy, they observed an increase in PDL-1 expression in lung tissue compared to the control mice. These findings suggest that PDL-1 expression is preferentially increased in lung tumour tissue by injecting LLC-GFP+ exosomes.
Researchers also examined the clinical significance of human TDEs and their manipulation of macrophages in the lymph nodes of non-small-cell lung cancer (NSCLC) patients. They found that there was increased expression of PDL-1 in the lymph nodes of these patients. To further study this, the researchers treated macrophages with either MLE-12 or LLC exosomes and then put them in a co-culture with OVA transgenic T-cells (a type of immune cell). The T-cells had a decrease in cell proliferation, which was later reversed by adding neutralizing α-PD-1 to the co-culture. This led them to think that PDL-1 had an inhibitory effect on T-cell function through macrophage stimulation. In summary, this study found that TDEs have an impact on PDL-1 expression found in tumour tissue and pre-metastatic tissue, resulting in creating an immunosuppressed environment for cancer cell growth. In my project, I’m interested in what cargo exosomes can carry from parental tumour cells to non-cancerous cells when creating a pre-metastatic niche. Knowing that there has been documented manipulation of the cellular makeup by exosomes encourages me to continue my research using neuroblastoma cells to elucidate the oncogenic markers relayed by exosomes. This research is very exciting, and I hope to shed some light on exosome impact in neuroblastoma tumour microenvironments with my own work!
Written by Alysia Scott









 The theme of the seminar was American Security: Integrating Multiple Perspectives. With the great support from North Carolina University, we stepped out our comfort zones and explored security topics through lenses of our cultural backgrounds and life experiences. Food Security, Energy Security and Environmental Security. Eighty-four Fulbrighters from almost 40 countries were sharing their stories on how these issues are dealt with in their home countries! We learnt to talk through being open-minded, find things in common and come to a balanced solution. This is not a-one-size-fits-all solution. We have more in common than we have thought. We have become friends and partners who build bridges and connect people and countries. The Fulbright Programme helped us to realise it!
The theme of the seminar was American Security: Integrating Multiple Perspectives. With the great support from North Carolina University, we stepped out our comfort zones and explored security topics through lenses of our cultural backgrounds and life experiences. Food Security, Energy Security and Environmental Security. Eighty-four Fulbrighters from almost 40 countries were sharing their stories on how these issues are dealt with in their home countries! We learnt to talk through being open-minded, find things in common and come to a balanced solution. This is not a-one-size-fits-all solution. We have more in common than we have thought. We have become friends and partners who build bridges and connect people and countries. The Fulbright Programme helped us to realise it!

 On November 22, almost all Americans and visitors celebrated
On November 22, almost all Americans and visitors celebrated 

 Barnes & Noble bookstore is located in the former Power Plant. The features are easily spotted. From outside, the building looks like a Plant for modern social activities. Ugly slightly, isn’t it? Though, it is a different feeling when you enter the bookstore. The Plant scaffolds, chimneys and pipes are nicely crafted into a warm welcoming environment. Even lights are dimmed as back then. Rambling through the bookshelves and feeling the magic of the place and unread stories on them. You can pick up a book, sit where you are and enjoy the reading. Maybe it is the feeling of my childhood full of books and hours of reading?
Barnes & Noble bookstore is located in the former Power Plant. The features are easily spotted. From outside, the building looks like a Plant for modern social activities. Ugly slightly, isn’t it? Though, it is a different feeling when you enter the bookstore. The Plant scaffolds, chimneys and pipes are nicely crafted into a warm welcoming environment. Even lights are dimmed as back then. Rambling through the bookshelves and feeling the magic of the place and unread stories on them. You can pick up a book, sit where you are and enjoy the reading. Maybe it is the feeling of my childhood full of books and hours of reading?