It’s the second round of journal club blog posts, and this time around, we’ll be looking at papers published by this very lab. I’ll be focussing on the paper in which the cell line I am currently working with (KellyCis83) was developed: “The development of cisplatin resistance in neuroblastoma is accompanied by epithelial to mesenchymal transition in vitro” This research addresses a critical challenge in cancer treatment: drug resistance, of course focusing on neuroblastoma, pediatric cancer notorious for its aggressive nature and poor prognosis that this lab has been studying for years.
Neuroblastoma is typically treated with cisplatin, a potent chemotherapy drug that induces DNA damage in cancer cells, leading to their death. The issue is that over time and particularly during relapse, some neuroblastoma cells develop resistance to cisplatin, rendering the treatment ineffective. Understanding the mechanisms behind this resistance is crucial for developing new therapeutic strategies.
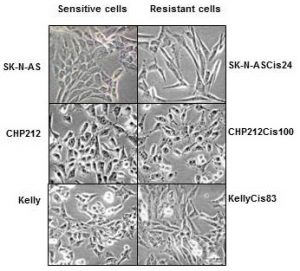
In this study, neuroblastoma cell lines resistant to cisplatin were created by gradually exposing the cells to increasing drug concentrations over 6 months. This approach mimics the clinical scenario where tumours are exposed to chemotherapy over an extended period, eventually leading to resistance. The resistant cell lines were then characterized to uncover the molecular changes that had occurred alongside or as part of the increased drug-resistance.
The cisplatin-resistant neuroblastoma cells exhibited significant disruptions in their cell cycle regulation, as highlighted by the most altered pathways identified by mass spectrometry. Cisplatin typically causes DNA damage that halts the cell cycle, leading to cell death. However, the researchers found upregulated pathways in resistant cells that allowed these cells to bypass this damage-induced arrest. One key finding was the identification of Vimentin upregulation in the upstream regulator analysis. Vimentin is a marker typically associated with epithelial-to-mesenchymal transition (EMT).
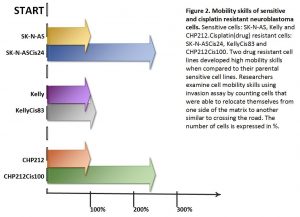
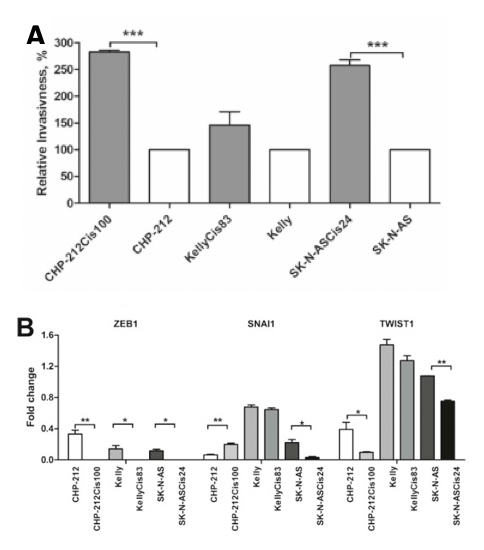
EMT is a process where epithelial cells acquire mesenchymal, fibroblast-like properties, including enhanced motility and apoptosis resistance. The link between EMT and cancer progression is well-established, as EMT not only facilitates metastasis but also contributes to drug resistance. In the context of neuroblastoma, the upregulation of Vimentin and dysregulation of related EMT proteins found in two of the resistant cell lines (specifically SNAI1 and TWIST1) suggests that these cells are not only evading cisplatin-induced cell cycle arrest but are also acquiring more aggressive, invasive characteristics. This links back to their findings on invasiveness, which showed greater levels in the two resistant cell lines that also had greater changes in EMT-related proteins (Figure 1).
Understanding the role of EMT in cisplatin-resistance opens up new avenues for therapeutic intervention. Targeting EMT-related pathways Vimentin could potentially restore the sensitivity of these resistant neuroblastoma cells to cisplatin, by targeting the evasive mechanisms the cells developed to bypass the cell-cycle disruption. Such therapies would offer a new strategy to tackle drug-resistant relapse cases, which currently have very poor outcomes.
Overall, this study provides a valuable model for investigating drug resistance in neuroblastoma and highlights the crucial role of EMT and its associated pathways in finding ways to treat drug-resistant tumours. As we continue to explore these avenues, these models will serve us as a strong foundation facilitating the research currently taking place in our lab towards finding such combination therapies and hopefully improving outcomes for children battling this devastating cancer in the future.
Written By Ronja Struck

 This was our 2nd time attending the OLCHC Research & Audit Day on May 25th, 2018. The conference provides a great forum for paediatric clinicians to share and update knowledge across different specialties through talks and poster presentations. It is insightful for basic biomedical researchers like us to see other perspectives.
This was our 2nd time attending the OLCHC Research & Audit Day on May 25th, 2018. The conference provides a great forum for paediatric clinicians to share and update knowledge across different specialties through talks and poster presentations. It is insightful for basic biomedical researchers like us to see other perspectives.