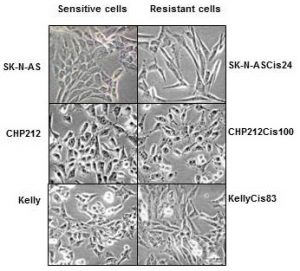
For children who do survive cancer, the battle is rarely over. Over 60% of long‐term childhood cancer survivors have a chronic illness as a consequence of the treatment they received; over 25% have a severe or life‐ threatening illness. How much do we know about quality of life of childhood cancer survivors?
Researchers in health- and illness-related social sciences understand that the there is a life after the treatment completed. The life is full if diverse levels and issues from health related to social adaptation in different shapes and forms. Children and teenagers may experience fear when returning to school due to temporary or permanent changes to their physical appearance (1,2). They worry about their ability to socialise with their friends due to lengthy absences (3–5). Treatment can result in the development of learning disabilities in children and thus marking school as a major source of frustration (1,2). These learning difficulties can affect a child’s confidence and self-esteem, if left without attention and care (1,3). All studies come to the same conclusion. Challenges in education of children with cancer are complex, however most can be tackled efficiently through planning and good communication (1–5).
Recently researchers working in FRED HUTCH Cancer Research Center asked adult childhood cancer survivors a number of health related questions about the quality of lives (6,7). The results are far from optimistic: “chance of surviving childhood cancer has improved — but survivors’ overall health has not”. You can find more by following the link.
It is important not only to recognise the problems but to start changing the situation. Apparently much more could be done more efficiently if patients are involved in setting up future research agenda.
Reading
- Gurney JG, Krull KR, Kadan-Lottick N, Nicholson HS, Nathan PC, Zebrack B, et al. Social outcomes in the childhood cancer survivor study cohort. J Clin Oncol. 2009;27(14):2390–5.
- McDougall J, Tsonis M. Quality of life in survivors of childhood cancer: A systematic review of the literature (2001-2008). Supportive Care in Cancer. 2009. p. 1231–46.
- Barrera M, Shaw AK, Speechley KN, Maunsell E, Pogany L. Educational and social late effects of childhood cancer and related clinical, personal and familial characteristics. Cancer. 2005;104(8):1751–60.
- Langeveld NE, Stam H, Grootenhuis MA, Last BF. Quality of life in young adult survivors of childhood cancer. Support Care Cancer. 2002;10(8):579–600.
- Klassen AF, Anthony SJ, Khan A, Sung L, Klaassen R. Identifying determinants of quality of life of children with cancer and childhood cancer survivors: A systematic review. Support Care Cancer. 2011;19(9):1275–87.
- Yeh JM, Hanmer J, Ward ZJ, Leisenring WM, Armstrong GT, Hudson MM, et al. Chronic Conditions and Utility-Based Health-Related Quality of Life in Adult Childhood Cancer Survivors. J Natl Cancer Inst [Internet]. 2016;108(9):4–7.
- Armstrong GT, Chen Y, Yasui Y, Leisenring W, Gibson TM, Mertens AC, et al. Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer. N Engl J Med. 2016;374(9):833–42.