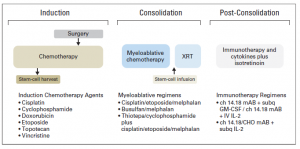
I was giving a talk at Georg-Speyer-Haus Institute for Tumour Biology and Experimental Therapy yesterday. The aim of my visit was to establish collaboration with Prof Daniela Krause, who is the expert in bone marrow microenvironment and targeted therapies. She took me to the Institute museum that keeps the history of this place and phenomenal researchers used to work there.
I was giving a talk at Georg-Speyer-Haus Institute for Tumour Biology and Experimental Therapy yesterday. The aim of my visit was to establish collaboration with Prof Daniela Krause, who is the expert in bone marrow microenvironment and targeted therapies. She took me to the Institute museum that keeps the history of this place and phenomenal researchers used to work there.
This research institute was established in 1904 to support work of Paul Ehrlich, its first director and funded by the private foundation “Chemotherapeutisches Forschungsinstitut Georg-Speyer-Haus”. Paul Erlich is the Father of the chemotherapy concept originally developed to treat diseases of bacterial origin. He reasoned that there should be a chemical compound that can specifically target bacteria and stop its growth. He developed Salvarsan, the most effective drug for treatment of syphilis until penicillin came onto the market.
Paul Erlich is also known for his contribution to cancer research. He and his colleagues actively experimented on how tumour originates and spread. They also tried to understand how immune system can beat cancer applying vaccination concepts.

Paul Erlich and Ilya Mechnikov were jointly awarded The Nobel Prize in Physiology or Medicine for his “work on immunity” in 1908.