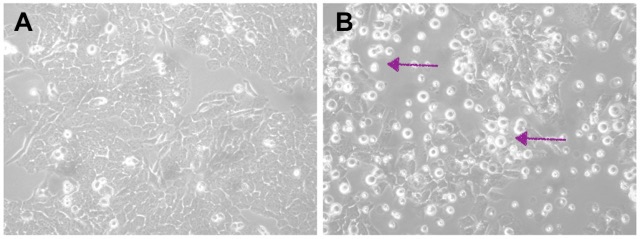
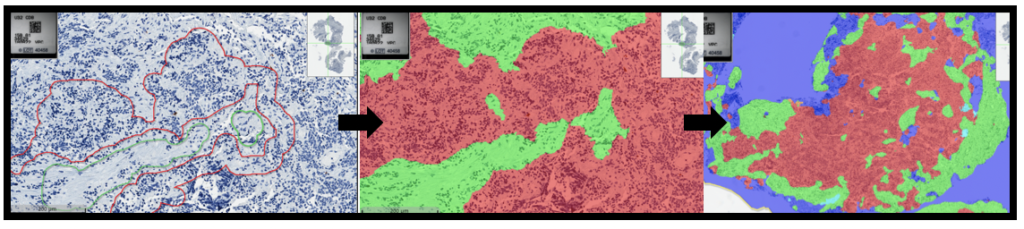
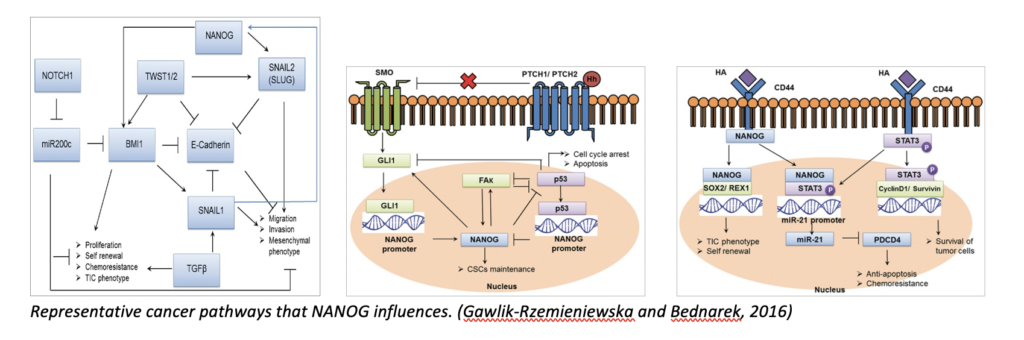
In humans, NANOG, SOX2, and OCT4 are transcription factors that maintain the undifferentiated state of embryonic stem cells (ESCs). NANOG was first discovered in 2003 by Chambers et al. and Mitsui et al. as a transcription factor in ESCs responsible for cellular self-renewal. More importantly, it enables continuous self-renewal of cancer stem cells, leading to metastasis when the regulatory genes involved do not function normally. These have been identified as cancer stem cells, with NANOG being a marker of “stemness”. In multiple cancer types, NANOG has various effects, including cellular expression of mesenchymal phenotype, cellular invasion/migration, repressed apoptosis, drug resistance, and increased angiogenesis. In pathways, NANOG either promotes or represses the expression of other genes that lead to cancer-favoured cellular behaviour. Overall, a higher expression level of NANOG is usually indicated in cases of poor prognosis.
NANOG is even more interesting due to its eponym, which comes from Tìr na nÒg. A Celtic myth of the Land of Youth, where the Tuath Dé resided in a supernatural land of paradise. This land offered beauty, health, joy, and everlasting youth to the inhabitants. As the myth goes, the Tuath Dé were gods of the land, and the god that ruled, Manannán mac Lir, was the first ancestor of humans. In various Celtic legends, humans are invited by the gods to visit Tìr na nÒg on great adventures.
However, time passes much slower in Tìr na nÒg, making it precarious for humans to return to their own world. As is the fateful tale of Oisín, who fell in love with the Tìr na nÒg goddess, Niamh. He travelled with her to Tìr na nÒg, where they lived happily in paradise. Upon a visit back to Ireland, Oisín realized that all his family had died over the years. When Oisín found a group of men who were struggling to move a giant rock, he stopped to lend them a hand while on his horse. However, the weight of the rock caused his saddle strap to snap. He fell from his horse, and when he touched the ground, he suddenly aged 300 years all at once.
Written by Alysia Scott
Sources:
Gawlik-Rzemieniewska, Natalia, and Ilona Bednarek. “The role of NANOG transcriptional factor in the development of malignant phenotype of cancer cells.” Cancer biology & therapy vol. 17,1 (2016): 1-10.
“The Story of Tír Na NÓg.” Celtic Titles, 10 Feb. 2022