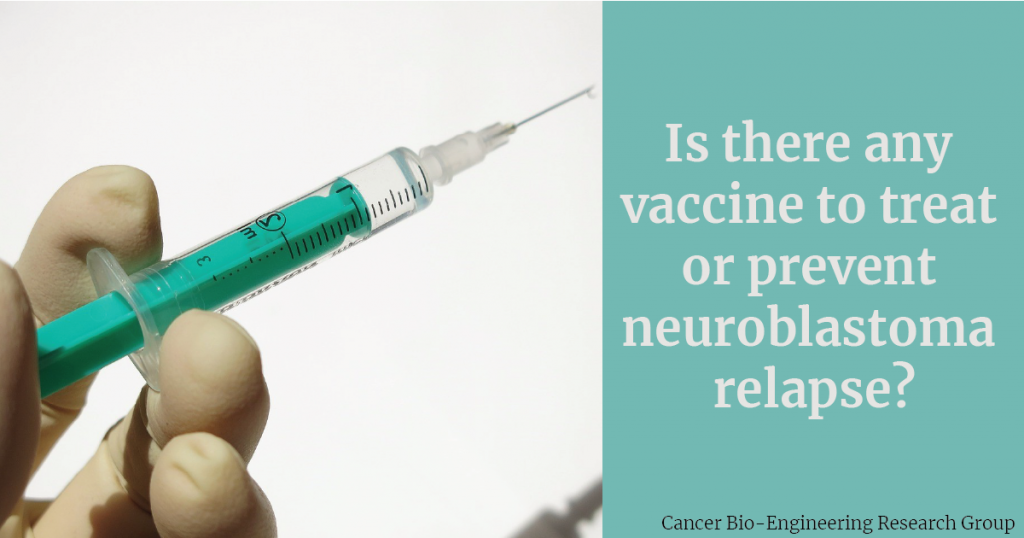
Anti-cancer vaccines teach the body’s immune system to identify and attack tumour cells. They are a type of immunotherapy and can be used to treat cancer or prevent tumour recurrence. Therefore, they are typically used in patients that have already received other treatments such as surgery, chemotherapy or radiotherapy.
Although anti-cancer vaccines have been gaining more attention over the years, few are being developed for paediatric tumours. From 594 clinical trials in neuroblastoma at clinicaltrials.gov, only 12 active trials are evaluating vaccines. Furthermore, these vaccines are still considered investigational products. They do not have the approval for use granted by health authorities. Therefore, these drugs are available for patients that enter into clinical trials.
An example of these vaccines is the bivalent vaccine for high-risk neuroblastoma developed in the Memorial Sloan Kettering Cancer Center in the US, collaborating with the biopharmaceutical company Y-mAbs Therapeutics. This vaccine is called bivalent because it has two proteins specifically present on the surface of neuroblastoma cells.
The rationale behind the treatment using this vaccine is that the body will be stimulated to produce antibodies against these two proteins. These antibodies will recognise and attach to neuroblastoma cancer cells, thus signalling to the immune system that these cells need to be eliminated.
A phase II trial evaluates vaccine efficacy in 374 patients who received seven subcutaneous injections of the vaccine in combination with an oral intake of an adjuvant, called β-glucan, that boosts the immune system1. The adjuvant intake started either on the first vaccine injection or on the third injection every two weeks until the end of the vaccine schedule. The study aims to analyse the anti-tumour effect of the vaccine and the immune response generated by the vaccine plus β-glucan therapy. The study is estimated to be completed by 2023.
The trials active for neuroblastoma vaccines are phase I or II. After these phases, there are still phases III and IV to complete the evaluation and continue monitoring these therapies. Therefore, in a few more years, we will know if neuroblastoma vaccines will be successful or not.
Written by Luiza Erthal
Reference
1. Memorial Sloan Kettering Cancer Center. Phase I/II Trial of a Bivalent Vaccine With Escalating Doses of the Immunological Adjuvant OPT-821, in Combination With Oral β-glucan for High-Risk Neuroblastoma. https://clinicaltrials.gov/ct2/show/NCT00911560 (2021).