The European Association for Cancer Research (EACR) is a registered charity and scientific community that has been holding conferences since 1968. EACR’s annual four-day congress is dedicated to basic, preclinical and translational cancer research. It brings together the cancer research community, including PhD students, postdocs, PIs, and commercial sponsors, for the opportunity to network and collaborate to progress cancer therapeutics.
I was fortunate enough to receive the Breakthrough Cancer Research Education and Travel Award, which made it possible for me to attend this year’s EACR conference held in Lisbon, Portugal. Breakthrough Cancer Research is an Irish Medical research charity focused on improving the outcomes of patients diagnosed with rare and poor prognosis cancers, like neuroblastoma.
When I first arrived at the congress center in Lisbon, I was immediately impressed by how well organized and put together the conference was. A schedule of four full days included speakers, poster presentations, industry talks, a technology exhibition, giveaways, networking rounds, and early-career talks. I checked in, received my “goodie bag” and was on my way to the first talk. For the duration of the conference, you were encouraged to move freely between all the available presentations within several auditoriums and pavilions. They even had screens and speakers set up outside the auditoriums if there was no more space inside to make sure that the research presented was accessible to everyone. The lunch breaks were the perfect time to enjoy the sunshine, walk along the Tagus River, and have a picnic with views of the Ponte 25 de Abril bridge (similar in style to the Golden Gate Bridge in San Francisco, California).

Exhibitors showcase with over 100 companies available to talk about their technology. QR codes were at each booth to scan for participants to be entered into a drawing for an iPad and free entry to next year’s conference in Budapest, Hungary.
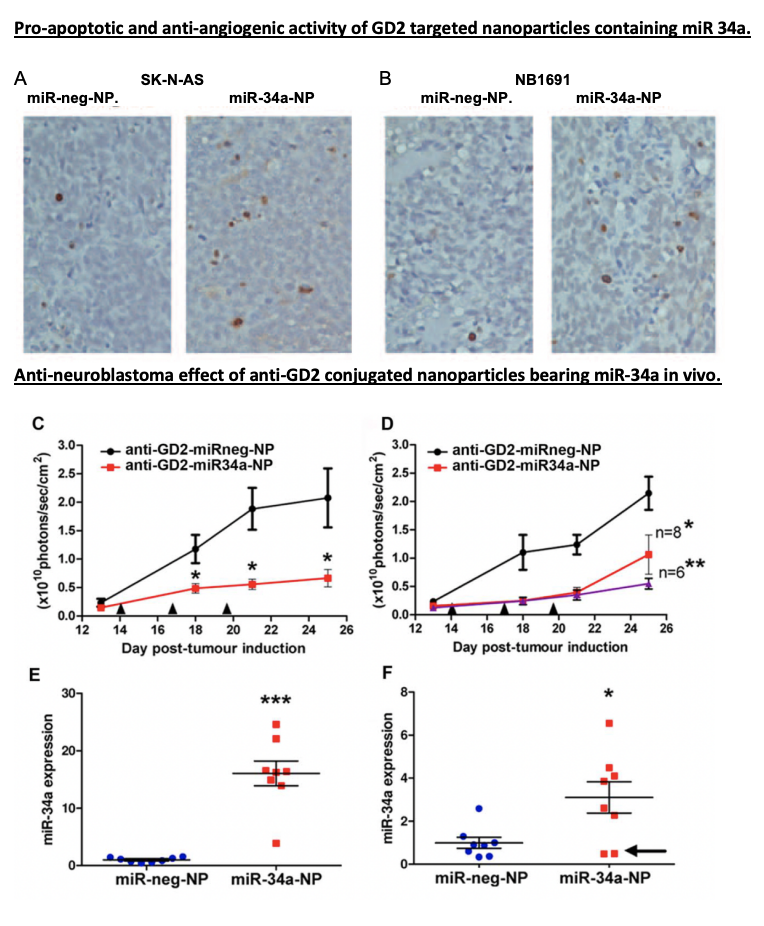
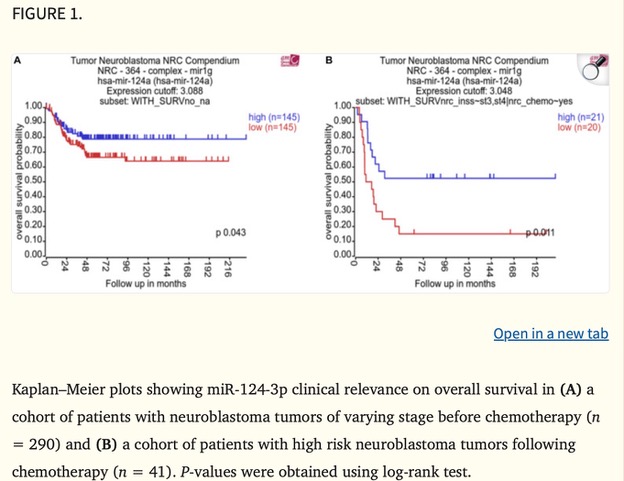
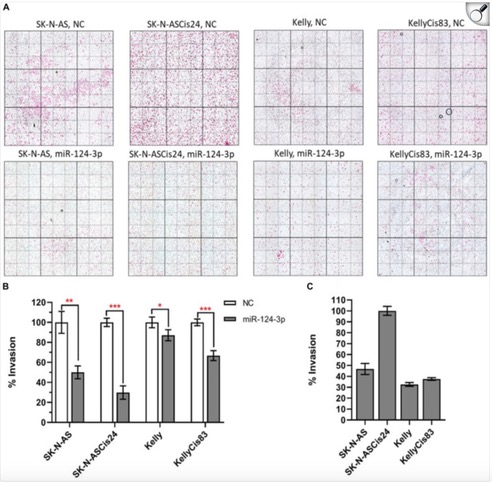
Throughout the conference, I listened to talks that ranged from how estrogen levels in breast cancer are related to the loss of bone density to how we can detect cancer in circulating cells for a diagnosis three years earlier than previous tests. One of the talks began with the necessity for physiologically relevant in vitro to 3D models and then the conclusion of the talk discussed how there’s a bridge needed between academia and industry for treatments to be more streamlined and accessible. Most importantly, I was able to read quite a few posters with research that other PhD students were doing related to small extracellular vesicles (sEVs). My work specifically looks at the relationship between sEVs shared from cancerous to non-cancerous cells and what their functional impact is. A lot of the work I saw was optimization of sEV isolation and characterization, which can be quite tricky to do but was helpful to see what complications others were running into and their troubleshooting results.
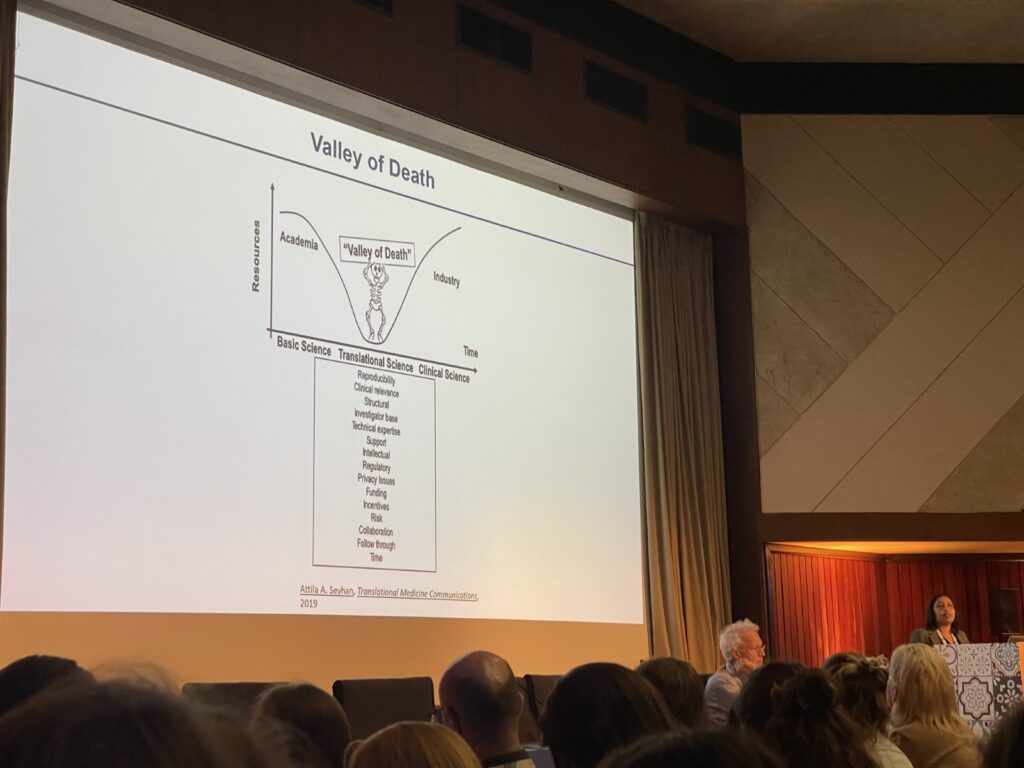
Presentation by Cindrilla Chumduri during EACR – EMBO Symposium: Advanced in vitro Models. Chumduri highlights the “valley of death” where there is a gap between academic and industry research that impedes the progression of scientific breakthroughs in cancer research.
By the time it got to my poster defense, I was excited to talk about my work and looking forward to meeting others who might be doing research similar to mine. There were a handful of people that came to speak to me about my work and ask questions. One thing about the PhD journey is that sometimes you can be so deeply involved in your own work and what isn’t going right that you lose sight of how impactful your work can be. When several people approached me about the co-culture model I was using, they were so curious and wanted to implement something like that into their work. Hearing positive feedback on my efforts was a refreshing way to end the conference. At the end of the day, there was a celebration dinner where a traditional Portuguese Fado band played music while we were able to unwind and network with other PhD students. My time spent in Lisbon at EACR was one of the best conference experiences I’ve had. I’m looking forward to heading back into the lab, making progress with my project, and presenting at the next conference.
My poster defense during the Tumor Biology poster sessions.
Special thanks to Breakthrough Cancer Research for supporting my research and providing me with this fantastic opportunity.
Written by Alysia Scott