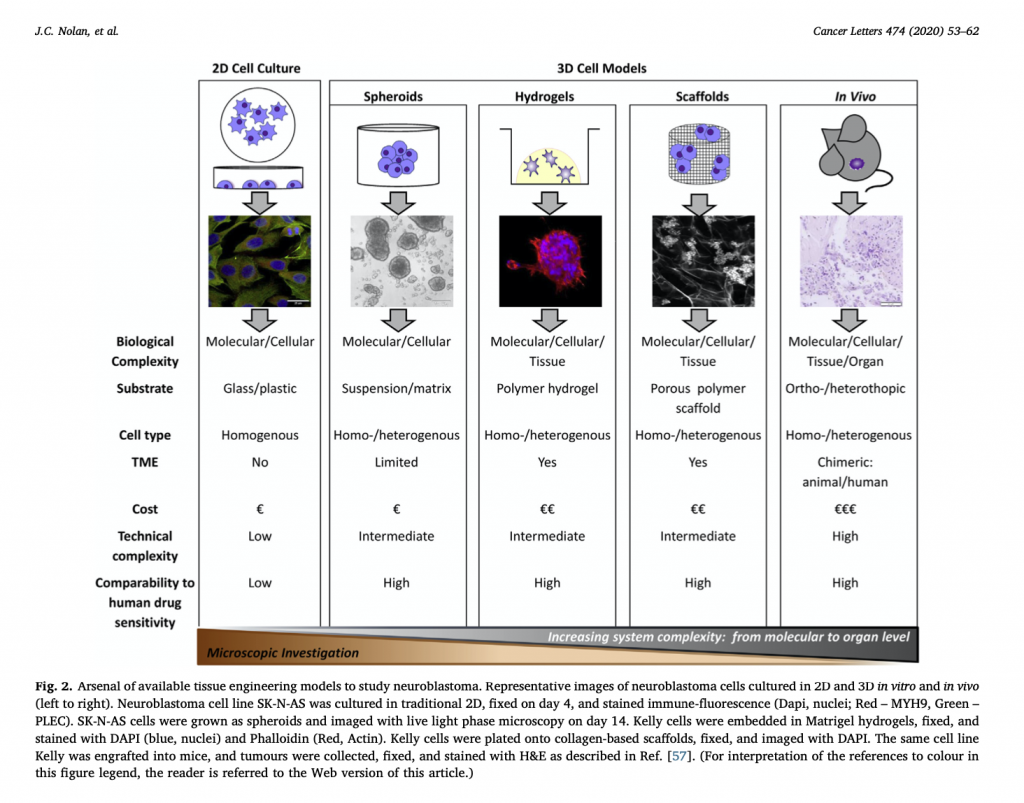
We’ve recently started a new journal club series focusing on papers published by our research group over the past few years. The paper I chose is titled “A Context-Dependent Role for MiR-124-3p on Cell Phenotype, Viability and Chemosensitivity in Neuroblastoma in vitro“. It explores the anti-cancer potential of miR-124-3p in neuroblastoma.
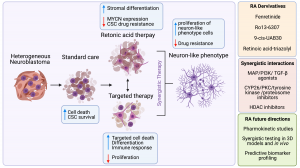
Neuroblastoma is particularly challenging to treat, especially when tumours become resistant to chemotherapy. This resistance is compounded by tumour heterogeneity—these cancers comprise different cell types, specifically adrenergic and mesenchymal cells. This variability affects treatment responses and plays a role in metastasis and how aggressively the cancer can spread.
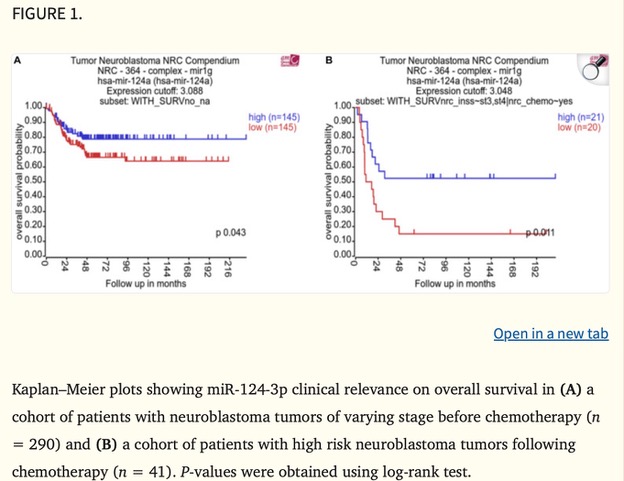
MicroRNAs (miRNAs) are small RNA molecules that regulate gene expression, and miR-124-3p has emerged as a promising player in cancer research. A Kaplan–Meier plot in the study (Figure 1) shows a strong association between low miR-124-3p levels and poorer survival rates in neuroblastoma patients, underscoring its potential impact on patient outcomes.
Our group’s study specifically examined how miR-124-3p might help reverse chemotherapy resistance and inhibit tumour cell growth in neuroblastoma. Excitingly, it has the potential to reduce cancer cell survival and increase their sensitivity to chemotherapy—an important breakthrough for treating resistant neuroblastomas.
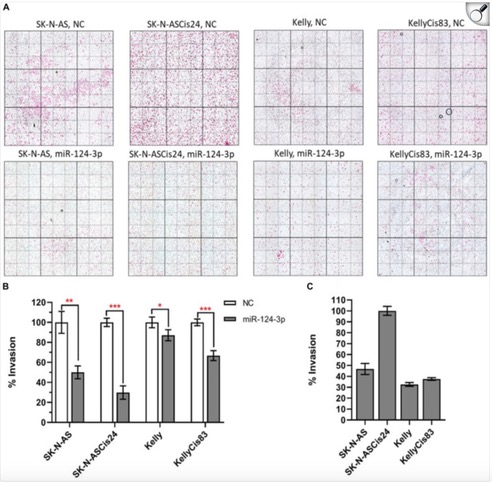
The study found that miR-124-3p directly targets genes involved in the epithelial-to-mesenchymal transition (EMT), a process that makes cancer cells more invasive and treatment-resistant. By suppressing these genes, miR-124-3p can reverse EMT, shifting cells to a less aggressive, more treatment-sensitive state. Our group observed that increased miR-124-3p significantly reduced neuroblastoma cell invasion (Figure 2). In SK-N-AS cells and their drug-resistant form, invasion dropped by 50% and 70%. In Kelly cells and their resistant form, invasion decreased by 10% and 30%. The most invasive of all, the drug-resistant SK-N-ASCis24 cells, showed the most substantial decrease in invasion after miR-124-3p treatment. This suggests that miR-124-3p could help limit neuroblastoma spread, highlighting its therapeutic potential.
While miR-124-3p isn’t part of my project, seeing how different molecular mechanisms can be harnessed to develop cancer therapies is always inspiring. Using miRNAs to sensitize resistant cancer cells to treatment could complement approaches like immunotherapies or vaccines, like the one I’m working on. Understanding these molecular pathways brings fresh perspectives on weakening cancer cells and making treatments more effective.
Written by Federica Cottone






 This was our 2nd time attending the OLCHC Research & Audit Day on May 25th, 2018. The conference provides a great forum for paediatric clinicians to share and update knowledge across different specialties through talks and poster presentations. It is insightful for basic biomedical researchers like us to see other perspectives.
This was our 2nd time attending the OLCHC Research & Audit Day on May 25th, 2018. The conference provides a great forum for paediatric clinicians to share and update knowledge across different specialties through talks and poster presentations. It is insightful for basic biomedical researchers like us to see other perspectives.