How do researchers study cells? How do we get the nitty gritty?
We use many methods to tag and chase various cell components. One of my favourites is fluorescent microscopy. It allows the use of nearly all spectrum of colours from blue to purple in one go. However, we prefer to narrow it down to 2-3 colours and avoid their overlap.
How does it work? First, we use DAPI or Hoescht, which are blue fluorescent dyes used to stain DNA. This way, we tag the nucleus of the cell. Then, we tag a protein of interest. In our case, it was MYCN, a protein that acts as a transcription factor. MYCN amplification is associated with poor prognosis in neuroblastoma. As a transcription factor, it binds to genomic DNA and is located in the nucleus. We used a specific antibody that was labelled with a green fluorescent dye. Look at the image below. The green colour pattern overlaps with the blue colour. Then, we tagged the cytoskeleton, a complex of various proteins that hold the cell architecture and dynamics. We used phalloidin with red fluorescence. It is a highly selective bicyclic peptide and a popular choice for staining actin filaments.
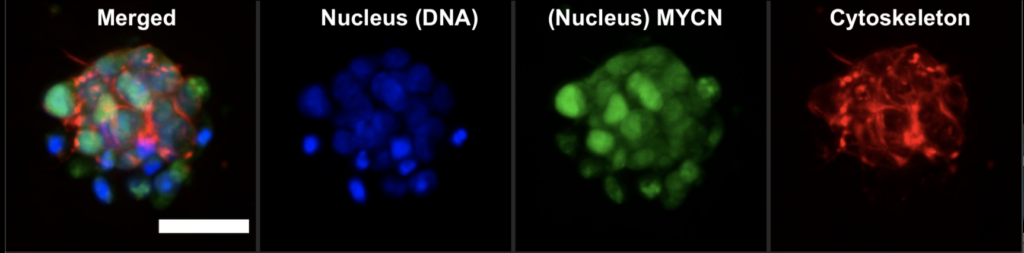
Now, we can enjoy visualising cells and test different research questions. For example, how do cells respond to a drug? Or how do neuroblastoma cells spread?
Written by Olga Piskareva

