I’m excited to kick off my second-year PhD journey with a deeper dive into cancer research. This is my first blog post of the year, and I’m eager to share what’s sparking my curiosity. So, I came across a paper by Tivnan et al. (2012), which focused on the targeted delivery of microRNA-34a (miR-34a) using nanoparticles. What intrigued me most was how these nanoparticles are designed to deliver therapies straight to cancer cells. Neuroblastoma is a highly aggressive and difficult-to-treat tumour, so finding a way to target it without affecting healthy cells could be a breakthrough.
Here’s what makes this study so exciting: the team developed a nanoparticle system coated with anti-GD2, a molecule that recognizes and binds to GD2, a marker commonly found on neuroblastoma cells. Think of these GD2-coated nanoparticles as specialized delivery trucks with a precise address—they’re designed to deliver miR-34a.
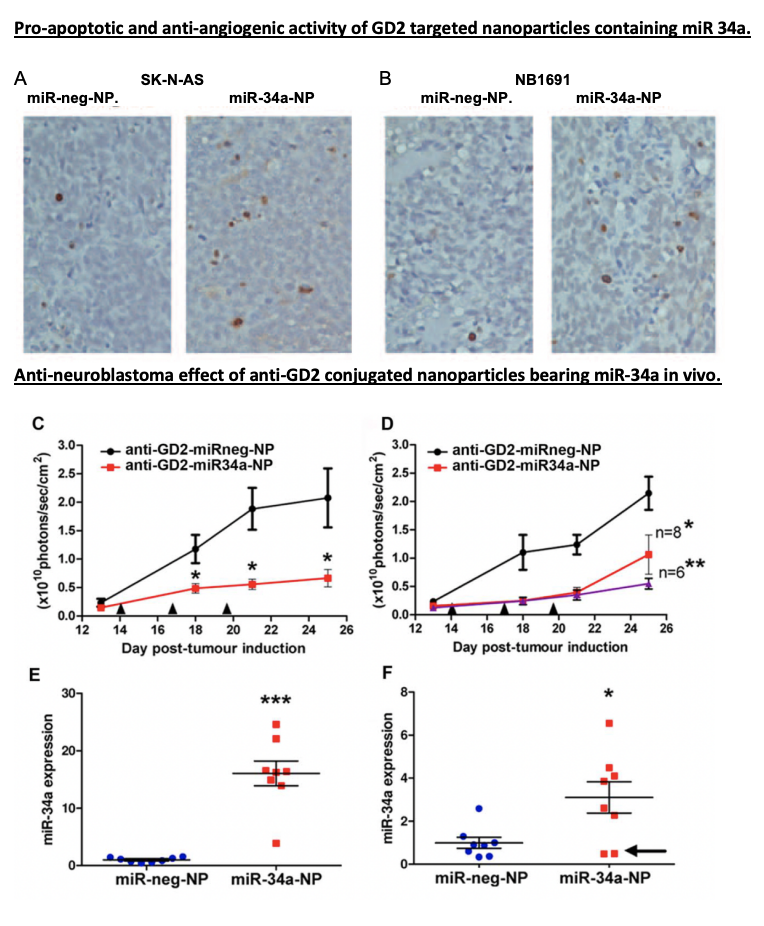
Now, let’s dive into the details of miR-34a’s role. MiR-34a isn’t just any therapeutic agent—it’s a master regulator capable of influencing multiple genes involved in cell growth, survival, and blood vessel formation. By releasing miR-34a into tumour cells, this study activated pathways that induced cell death and suppressed angiogenesis, preventing the tumour from forming new blood vessels. It’s almost as if miR-34a is a conductor orchestrating a complex, multi-step attack on cancer, using the tumour’s own cellular mechanisms against it.
The Results? A Direct and Multi-Layered Attack on Tumor’s
In their mouse model, the GD2-targeted nanoparticles packed with miR-34a significantly reduced tumour growth. These “smart” nanoparticles didn’t just shrink tumors by inducing apoptosis (cell death); they also cut off the tumor’s blood supply by promoting the expression of TIMP2, an anti-angiogenic protein. Essentially, the tumor cells were directly targeted and deprived of the resources they needed to survive—a powerful one-two punch.
Where Do We Go From Here?
This study is an excellent example of how targeted therapies could evolve to tackle other types of cancer. Traditional therapies, like chemotherapy, often affect both healthy and cancerous cells, leading to significant side effects. In contrast, this targeted approach delivers miR-34a specifically to neuroblastoma cells, which could be especially beneficial for pediatric patients who need treatments that minimize harm to developing bodies. Imagine pairing nanoparticles like these with different therapeutic targets, such as GPC2, ALK, or PDL1, or even combining them with existing treatments to boost effectiveness while minimizing side effects. For those in the field, the potential here feels like a breakthrough waiting to happen.
Written By Rabia Saleem

