This post is dedicated to parents of children with neuroblastoma. Some parents asked about DFMO – a re-purposing drug. In this post, I tried to collect and summarize information available from academic sources.
Q1: What is DFMO?
Difluoromethylornithine (DFMO, Eflornithine) is an anti-protozoan drug. It was originally developed and FDA approved for the treatment of Trypanosoma brucei gambiense encephalitis (“African sleeping sickness”). DFMO permanently binds to ornithine decarboxylase (ODC), an important enzyme in polyamine metabolism, and prevents the natural substrate ornithine from entering the active site.
By inhibiting ODC, DFMO reduces cellular polyamines and inhibits cell growth and proliferation of actively dividing cells, thus making DFMO an attractive candidate for cancer therapy. In neuroblastoma, a positive regulation of all aspects of polyamine metabolism by MYCN was reported (revived by Bassiri 2015, Gamble 2012). So, it is believed that MYCN amplified neuroblastomas would most benefit of the drug.
Q2: How intense is basic science behind DFMO in neuroblastoma?
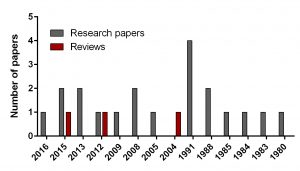
To find out the intensity of basic science on DFMO in neuroblastoma search for ‘difluoromethylornithine/DFMO/Eflornithine’ and ‘neuroblastoma’ was run in PubMed, a web-based resource with 26 million citations for biomedical literature from MEDLINE, life science journals, and online books. The search returned 23 papers including 3 reviews and 20 primary research reports published from 1980 to present.
In comparison, I did another search for a novel drug Unituxin (dinutuximab) approved by FDA in 2015. It is monoclonal antibody against the glycolipid disialoganglioside GD2, a biomarker specific for neuroblastoma. Search for ‘anti-GD2 antibody’ and ‘neuroblastoma’ returned 181 papers including 25 reviews and 156 primary articles for the same period.
Q3: Is DFMO in cancer clinical trials?
“ClinicalTrials.gov is a Web-based resource that provides patients, their family members, health care professionals, researchers, and the public with easy access to information on publicly and privately supported clinical studies on a wide range of diseases and conditions. The Web site is maintained by the National Library of Medicine (NLM) at the National Institutes of Health (NIH).
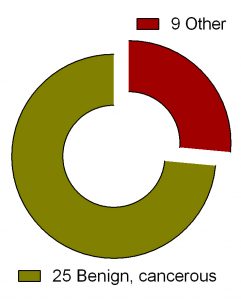
Search for ‘difluoromethylornithine/DFMO/Eflornithine’ in ClinicalTrials.gov returned 36 registered trials across different health conditions.Two of these were withdrawn, the breakdown for the rest 34 is as follows: Adenomatous Polyp (1), Anaplastic Astrocytoma/Recurrent Anaplastic Astrocytoma (1), Bladder Cancer (1), Cervical Cancer/Precancerous Condition (1), Colorectal Cancer (3), Esophageal Cancer (1), Familial Adenomatous Polyposis (1), Gastric Cancer/Gastric Intestinal Metaplasia (1), Hirsutism (2), Human African Trypanosomiasis (5), Neuroblastoma (7), Non-melanomatous Skin Cancer/Precancerous/Nonmalignant Condition (4), Post-solid Organ Transplant/Skin Neoplasms (1), Precancerous Condition (1), Prostate Cancer (2), Pseudofolliculitis Barbae (1), Type 1 Diabetes (1) (Fig. 2). To see full details of 34 trials please click at this Table.
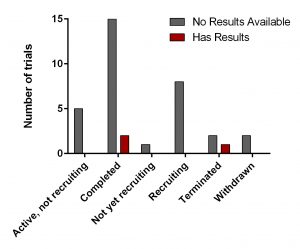
All of them have various statuses (Fig. 3) as well as study design. Importantly, 30 out of 34 studies are focused on safety and efficacy of this drug. Vast majority of studies of DFMO in adult cancers/benign conditions are randomized (16/18 or 89%). Randomization in assignment of patients in studied groups (control and new drug/combination) helps minimize researcher’s bias when comparing effect of the new treatment vs current/no treatment. All trials of DFMO in neuroblastoma are not randomized. Instead, studies use a single group assignment.
Three trails have been either completed/terminated and published results are available at ClinicalTrials.gov (NCT01059071, NCT00033371. NCT00118365).
Q4: What about clinical trials of DFMO in neuroblastoma?
Seven clinical trials on DFMO in neuroblastoma are registered with ClinicalTrials.gov (NCT01586260, NCT02395666, NCT01059071, NCT02679144, NCT02139397, NCT02030964, NCT02559778). One of these has been completed and results are available – ‘Safety study for refractory or relapsed neuroblastoma with DFMO alone and in combination with etoposide (NCT01059071)’.
The trial NCT01059071 was a Phase 1 clinical trial. A phase I clinical trial tries to find out whether a new treatment/drug is safe, what its side effects are, the best dose of the new treatment, if the treatment shrinks the cancer.
Twenty one patients were enrolled and eligible for treatment with DFMO and DFMO + etoposide. These patients were assigned into 4 groups of different DFMO doses (Fig. 4). The treatment was in cycles of 21 days. Cycle 1 – DFMO only followed by cycle 2 – combined treatment of DFMO+etoposide (14 days) and DFMO only (the last 7 days).
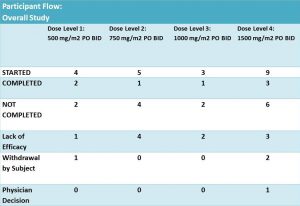
According to results of the trial: 14 patients did not complete the treatment due to different reasons. It was not clear what stage/cycle they left the trial.
More details on this trial were provided in the corresponding paper ‘A Phase I Trial of DFMO Targeting Polyamine Addiction in Patients with Relapsed/Refractory Neuroblastoma‘ published in PLOS ONE. PLOS ONE is peer-reviewed, open-access online resource reporting scientific studies from all disciplines. .
Q5: How the study was designed?
As mentioned earlier this study used a single group assignment and a design called ‘3+3’. This design is straightforward and safe. Briefly, it means that for a dose (X) of the drug, 6 patients are selected. Of these, 3 receive the dose X and are monitored for a period of time. If no adverse effects are registered in these 3, then another new 3 patients start the same treatment. The effect of the drug is evaluated on the patent’s health condition before-, during – the treatment and after its completion. This approach is often used in vaccine tests and dose escalation methods in Phase I cancer clinical trials. This type of study can answer mainly two questions: 1) whether the tested drug is safe to use and 2) what doses are safe? The main drawbacks of this design are
- Many patients treated at doses below therapeutic effect
- Slow dose increase
- Uncertainty about the recommended phase II dose (RP2D)
- Only the result from the current dose is used for determining the dose of next cohort of patients. Information on other doses is ignored
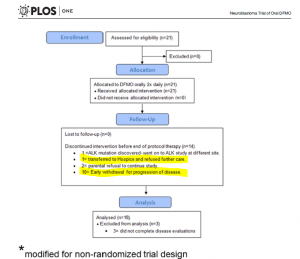
Q6: What are main findings of the clinical trial NCT01059071?
The overflow of the study is presented in Fig 5 providing additional information on those who did not complete the trial. Out of 14 participants, disease has progressed in 11 patients – it is 52% of the enrolled participants. Authors highlighted that this phase I study was not designed to evaluate anti-tumour efficacy of DFMO. But tumour response and clinical response were monitored during the study.
According to the paper, 21 patients received at least one dose of DFMO only (Cycle 1, 21 days). During this cycle, 3 patients were withdrawn. All of them were assessed for safety of DFMO.
Adverse effects in response to DFMO alone were: anemia – 3, ANC decrease – 2, decreased platelet count – 2, ALT increase – 1, AST increase – 1, anorexia – 1, constipation – 1, diarrhea – 1, infection (conjunctivitis) – 1, hypoalbuminemia – 1, hypophosphatemia – 1, increased GGT – 1, sleep disturbance – 1, urinary retention – 1 and vomiting – 1.
Eighteen of them completed cycle 1 and continued treatment with DFMO+etoposide for another 4 cycles followed with DFMO only therapy for a number of cycles. Their clinical response data were examined for efficacy of DFMO alone.
Three out of 21 participating patients in this clinical trial remain alive and disease free between 2–4.5 years after starting DFMO.
Authors concluded that
DFMO doses of 500-1500mg/m2/day are safe and well tolerated in children with relapsed NB
Research and review papers covering DFMO in neuroblastoma:
- Evageliou NF, Haber M, Vu A, Laetsch TW, Murray J, Gamble LD, Cheng NC, Liu K, Reese M, Corrigan KA, Ziegler DS, Webber H, Hayes CS, Pawel B, Marshall GM, Zhao H, Gilmour SK, Norris MD, Hogarty MD. Polyamine Antagonist Therapies Inhibit Neuroblastoma Initiation and Progression. Clin Cancer Res. 2016 Sep 1;22(17):4391-404. doi: 10.1158/1078-0432.CCR-15-2539.
- Bassiri H, Benavides A, Haber M, Gilmour SK, Norris MD, Hogarty MD. Translational development of difluoromethylornithine (DFMO) for the treatment of neuroblastoma. Transl Pediatr. 2015 Jul;4(3):226-38. doi: 10.3978/j.issn.2224-4336.2015.04.06. Review.
- Saulnier Sholler GL, Gerner EW, Bergendahl G, MacArthur RB, VanderWerff A, Ashikaga T, Bond JP, Ferguson W, Roberts W, Wada RK, Eslin D, Kraveka JM, Kaplan J, Mitchell D, Parikh NS, Neville K, Sender L, Higgins T, Kawakita M, Hiramatsu K, Moriya SS, Bachmann AS. A Phase I Trial of DFMO Targeting Polyamine Addiction in Patients with Relapsed/Refractory Neuroblastoma. PLoS One. 2015 May 27;10(5):e0127246. doi: 10.1371/journal.pone.0127246.
- Lozier AM, Rich ME, Grawe AP, Peck AS, Zhao P, Chang AT, Bond JP, Sholler GS Targeting ornithine decarboxylase reverses the LIN28/Let-7 axis and inhibits glycolytic metabolism in neuroblastoma. Oncotarget. 2015 Jan 1;6(1):196-206.
- Samal K, Zhao P, Kendzicky A, Yco LP, McClung H, Gerner E, Burns M, Bachmann AS, Sholler G. AMXT-1501, a novel polyamine transport inhibitor, synergizes with DFMO in inhibiting neuroblastoma cell proliferation by targeting both ornithine decarboxylase and polyamine transport. Int J Cancer. 2013 Sep 15;133(6):1323-33. doi: 10.1002/ijc.28139.
- Koomoa DL, Geerts D, Lange I, Koster J, Pegg AE, Feith DJ, Bachmann AS. DFMO/eflornithine inhibits migration and invasion downstream of MYCN and involves p27Kip1 activity in neuroblastoma. Int J Oncol. 2013 Apr;42(4):1219-28. doi: 10.3892/ijo.2013.1835.
- Gamble LD, Hogarty MD, Liu X, Ziegler DS, Marshall G, Norris MD, Haber M. Polyamine pathway inhibition as a novel therapeutic approach to treating neuroblastoma. Front Oncol. 2012 Nov 16;2:162. doi: 10.3389/fonc.2012.00162. Review
- Passariello CL, Gottardi D, Cetrullo S, Zini M, Campana G, Tantini B, Pignatti C, Flamigni F, Guarnieri C, Caldarera CM, Stefanelli C. Evidence that AMP-activated protein kinase can negatively modulate ornithine decarboxylase activity in cardiac myoblasts. Biochim Biophys Acta. 2012 Apr;1823(4):800-7. doi: 10.1016/j.bbamcr.2011.12.013.
- Rounbehler RJ, Li W, Hall MA, Yang C, Fallahi M, Cleveland JL. Targeting ornithine decarboxylase impairs development of MYCN-amplified neuroblastoma. Cancer Res. 2009 Jan 15;69(2):547-53. doi: 10.1158/0008-5472.CAN-08-2968.
- Koomoa DL, Yco LP, Borsics T, Wallick CJ, Bachmann AS. Ornithine decarboxylase inhibition by alpha-difluoromethylornithine activates opposing signaling pathways via phosphorylation of both Akt/protein kinase B and p27Kip1 in neuroblastoma. Cancer Res. 2008 Dec 1;68(23):9825-31. doi: 10.1158/0008-5472.CAN-08-1865.
- Hogarty MD, Norris MD, Davis K, Liu X, Evageliou NF, Hayes CS, Pawel B, Guo R, Zhao H, Sekyere E, Keating J, Thomas W, Cheng NC, Murray J, Smith J, Sutton R, Venn N, London WB, Buxton A, Gilmour SK, Marshall GM, Haber M. ODC1 is a critical determinant of MYCN oncogenesis and a therapeutic target in neuroblastoma. Cancer Res. 2008 Dec 1;68(23):9735-45. doi: 10.1158/0008-5472.CAN-07-6866.
- Wallick CJ, Gamper I, Thorne M, Feith DJ, Takasaki KY, Wilson SM, Seki JA, Pegg AE, Byus CV, Bachmann AS. Key role for p27Kip1, retinoblastoma protein Rb, and MYCN in polyamine inhibitor-induced G1 cell cycle arrest in MYCN-amplified human neuroblastoma cells. Oncogene. 2005 Aug 25;24(36):5606-18.
- Bachmann AS. The role of polyamines in human cancer: prospects for drug combination therapies. Hawaii Med J. 2004 Dec;63(12):371-4. Review
- Chen ZP, Chen KY. Differentiation of a mouse neuroblastoma variant cell line whose ornithine decarboxylase gene has been amplified. Biochim Biophys Acta. 1991 Dec 3;1133(1):1-8.
- Piacentini M, Fesus L, Farrace MG, Ghibelli L, Piredda L, Melino G. The expression of “tissue” transglutaminase in two human cancer cell lines is related with the programmed cell death (apoptosis). Eur J Cell Biol. 1991 Apr;54(2):246-54.
- Melino G, Piacentini M, Patel K, Annicchiarico-Petruzzelli M, Piredda L, Kemshead JT. Retinoic acid and alpha-difluoromethylornithine induce different expression of neural-specific cell adhesion molecules in differentiating neuroblastoma cells. Prog Clin Biol Res. 1991;366:283-91.
- Stephanou A, Knight RA, De Laurenzi V, Melino G, Lightman SL.Expression of pre-opiomelanocortin (POMC) mRNA in undifferentiated and in vitro differentiated human neuroblastoma cell lines. Prog Clin Biol Res. 1991;366:173-80.
- Melino G, Farrace MG, Ceru’ MP, Piacentini M. Correlation between transglutaminase activity and polyamine levels in human neuroblastoma cells. Effect of retinoic acid and alpha-difluoromethylornithine. Exp Cell Res. 1988 Dec;179(2):429-45.
- Chen KY, Dou QP. NAD+ stimulated the spermidine-dependent hypusine formation on the 18 kDa protein in cytosolic lysates derived from NB-15 mouse neuroblastoma cells. FEBS Lett. 1988 Mar 14;229(2):325-8.
- Karvonen E, Andersson LC, Pösö H. A human neuroblastoma cell line with a stable ornithine decarboxylase in vivo and in vitro. Biochem Biophys Res Commun. 1985 Jan 16;126(1):96-102.
- Pösö H, Karvonen E, Suomalainen H, Andersson LC. A human neuroblastoma cell line with an altered ornithine decarboxylase. J Biol Chem. 1984 Oct 25;259(20):12307-10.
- Chen KY, Nau D, Liu AY. Effects of inhibitors of ornithine decarboxylase on the differentiation of mouse neuroblastoma cells. Cancer Res. 1983 Jun;43(6):2812-8.
- Chapman SK. Antitumor effects of vitamin A and inhibitors of ornithine decarboxylase in cultured neuroblastoma and glioma cells. Life Sci. 1980 Apr 21;26(16):1359-66. No abstract available.

