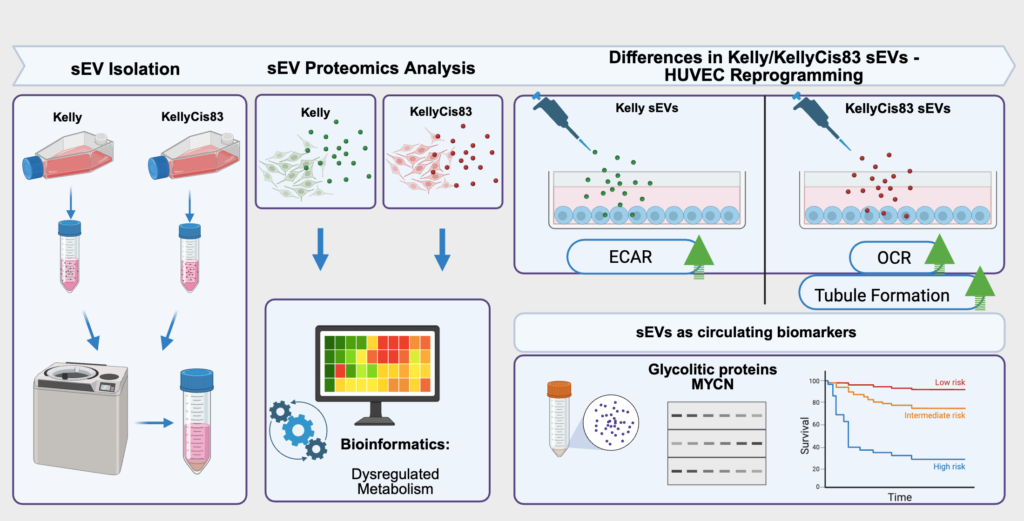
What a year – two young and talented postgraduate students have been minted with a Doctor of Philosophy Degree in September and December of 2025. They are Dr Lin Ma and Dr Ronja Struck. Hard work and dedication are the cornerstones of this challenging but rewarding journey.
They sailed through scattered showers and sunny spells, gale winds and stormy snow with sunshine developing elsewhere, turning chilly under clear skies on some days with temperatures below/above zero. The full spectrum of emotions and hard work was spiced up by the uncertainty of COVID-19 restrictions. Well done to Ronja and Lin!

My greatest thanks to Lin’s examiners Prof Sue Burchill (University of Leeds, UK), Dr Joan Ní Gabhann-Dromgoole (RCSI, Ireland) and the independent chair Prof Kevin McGuigan (RCSI, Ireland)!!

My greatest thanks to Ronja’s examiners, Prof Martina Rauner (Dresden University, Germany), Prof Fabio Quondamatteo (RCSI, Ireland) and the independent chair Dr Inmar Schoen (RCSI, Ireland)!!
This work would not be possible without the generous support from the Irish Research Council (Research Ireland) and the Conor Foley Neuroblastoma Cancer Research Foundation to Ronja, and from the RCSI-Soochow University StAR International PhD Programme to Lin.