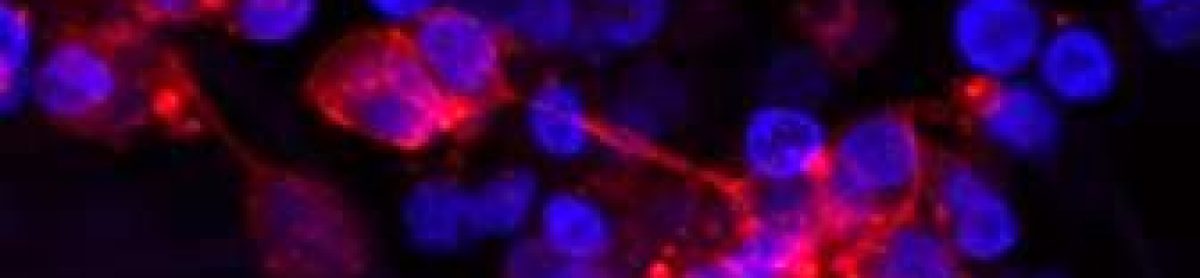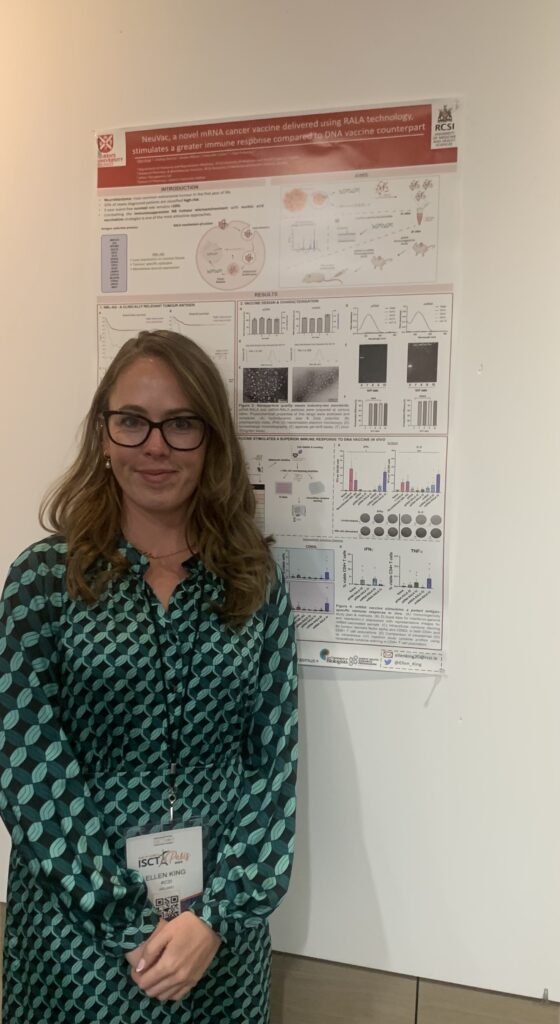
I’m Ellen, and I am a 3rd year PhD student in the Cancer Bioengineering Group. Last week I attended and presented at my first international conference, ISCT (International Society for Cell & Gene Therapy), in Paris. I spent five days in Paris with three of them at the conference where scientists, researchers and pharma professionals came from far and wide. There was a strong focus on collaboration between industry teams and academics, and it gave me a lot to think about when it comes to my own PhD and career journey as a whole.
As a soon-to-be final-year student, the next step in my career has been on my mind. Starting out, I was very sure I wanted to progress within academia and follow the “traditional” researcher route. Industry always seemed so far removed from the basic sciences, and specifically biology research roles are hard to come by in Ireland. Having the opportunity to travel to Paris and meet with such a wide range of professionals really opened my eyes to the possibility of a career in the industry. It was reassuring to see that even after leaving academia, there is a cross-over and lots of collaboration. Industry or academia? The fork in the road when it comes to this career choice is becoming lesser and lesser.
While I was in Paris, I had a lot of time to ponder the fantastic science and research that I discussed at the talks (Did you know? One adult human heart produces enough energy in one lifetime to power an 18-wheeler to the moon and back). Additionally, I could also see first-hand that the positive aspects that we associate with academia (presenting research, freedom of research topics and the conference wine receptions, of course) are also readily available as a non-academic based scientist. In fact, there is a career that has the “goodness of both”. So many academics discussed start-ups and spin-out companies developed off the back of their academic research, and there were even talks that discussed the how, what, when and where of transitioning between the two settings.

I’m so grateful that I could attend this conference. I presented my research (a project very much blended between academia and industry), got to chat to like-minded people and came home with a wealth of new knowledge. This knowledge will not only enrich my PhD project but will stand for me as my career moves from student to fully-fledged scientist. The topic of post-PhD job hunting often comes with a knot in the stomach, but seeing the exciting opportunities that are available out there has me much more excited than stressed about this next step. And now to finish this PhD so that I can take that next step 🙂
My trip became possible thanks to the Company of Biologist travel grant and support from the RCSI Department of Anatomy and Regenerative Medicine.
Written by Ellen King