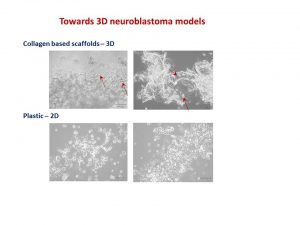
Our body has 3 dimensions: height, width and depth. Every single part of our body grows in the same 3 dimensions. This is true for cancer cells. Researchers use different ways to study cancer cells behaviour, how they grow and spread. We grow cells in the flasks, where they change their structure and shape and become flat losing one dimension. This is a very popular approach. We also grow cells in mice, where cells keep their 3D shape and mimic their behaviour to one observed in humans.
It is well known that we need to give a different amount of drug to kill cancer cells grown in flasks and in mice. This, in turn, delays the development of new drugs. Why does it happen this way? So, the drug works only on one side of the cell when they grow on the flat surface. In contrast, in mice, drug surrounds the cancer cell habitat and attacks cells at the edge first and then getting to those at the core. So we need more drug to kill cancer cells in mice.
We decided to design a new way to grow cancer cells that recreate their growth in 3 dimensions as in the human or mice body. We used special cotton wool like sponges as a new home for cancer cells and populated them with cancer cells. At the next step, we gave cells the drug at the different amount and checked what happened.
To understand cell fitness we stained them with red and blue dyes. On the left bottom side of the image, we see an equal amount of red and blue dyes telling us that cells were healthy and fit. Cells did not get any drug. When we gave a little amount of the drug but enough to kill cells in the flask, the balance of red and blue dyes was the same telling us that nothing really happened (the image in the middle). Cells were feeling well and healthy. The right bottom image has only blue dye. In this case, cells were given the amount of drug enough to destroy cancer cells in mice or humans. The lack of red dye tells us that this time the drug worked and killed the cancer cells.
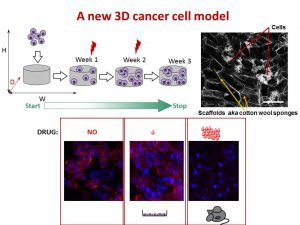
We found that the drug killed cells on sponges only at doses enough to do the same in mice.
So, we concluded the new tactic to grow cancer cells in 3D on cotton-like sponges can bridge the gap between traditional way and animal models. This new strategy to grow cells on sponges should help to understand cancer cell behaviour better and accelerate the discovery and development of new effective drugs for neuroblastoma and other cancers. This, in turn, will make the outlook for little patients better and improve their quality of life.
This work has been published in Acta Biomaterialia and presented recently at the Oral Posters Session at the 54th Irish Association for Cancer Research Conference 2018.
This study was supported by Neuroblastoma UK and National Children’s Research Centre.
You can find more at