Here are two pictures of young and promising researchers. Both are inspecting cells under the microscope. Can you spot 5 differences?
2. Head cover?
3. Microscope?
4. Lab coat?
5. ?

Blog about neuroblastoma research
My WordPress Blog is about neuroblastoma biology and Cancer Bioengineering Group
Here are two pictures of young and promising researchers. Both are inspecting cells under the microscope. Can you spot 5 differences?


Reading my posts, it looks like I am more enjoying the cultural part and almost forgot the main reason I crossed the Atlantic with the Fulbright wings.
The first month in the lab was more a warming up. Where is my desk? Where is the cell culture rooms? How do they run it? How different is it? So, many microscopes – am I capable of imaging? And so on and so forth…
My typical day starts at 8-8.30 am and finishes once all is done. It may be 6pm or 10pm. Once the experiment is set up, I have to monitor cells every 24 hours for 5-7 days with no weekends or days off. The monitoring includes imaging. Lots of imaging. Every condition has 20-30 single cells to follow up. Each cell gets its own GPS tag manually to be able to image exactly the same cell as it grows and becomes a group of hundreds by multiplication. For example, I am running 8 different cell lines in 3 experimental conditions. So, 20-30 cells per all 24 combinations give us 480-720 individual cells to follow up. The imaging takes ~5 hours every day. After 5 days, I will have 2400 – 3600 pics of cells to analyse. It will be fun! I may need lots of Guinness to fly through that numbers.
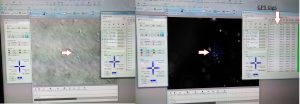
At the next step, I will select some of the conditions for video recording to trace cell fate from a single neuroblastoma cell to a metastatic niche consisting of hundreds of them. This video will show me how it all happens minute after minute.
Is not it exciting? I am thrilled!
Another year, another Research Summer School students. We are hosting 4 students (Jessica, Dawn, Dola, and Jeff) this year. Some of them will be medical doctors, another will do research after the graduation. For them, the 8-weeks lab placement is a window into the reality of the everyday science. How cancer cells look? How do they grow? Where do we store them? How do we know that we have identified a new drug or a new target to study further? Do researchers have a sense of humour? Do they like donuts?

We have already said Good Bye to Jessica. Dola and Dawn’s projects are coming to an end this week, while Jeff is staying till the end of August.
 This was our 2nd time attending the OLCHC Research & Audit Day on May 25th, 2018. The conference provides a great forum for paediatric clinicians to share and update knowledge across different specialties through talks and poster presentations. It is insightful for basic biomedical researchers like us to see other perspectives.
This was our 2nd time attending the OLCHC Research & Audit Day on May 25th, 2018. The conference provides a great forum for paediatric clinicians to share and update knowledge across different specialties through talks and poster presentations. It is insightful for basic biomedical researchers like us to see other perspectives.
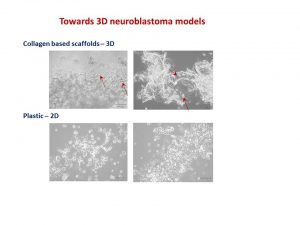
I was delighted to know that two our studies were shortlisted. It is a rewarding feeling to see your Dream Team doing very well. One was the project of the Erasmus+ student Hanne Pappaert and the other was the project of NCRC funded Postdoc John Nolan. Hanne explored our 3D tissue-engineered model of neuroblastoma using collagen-based scaffolds with distinct mechanical properties. These new scaffolds were designed and manufactured by our collaborator Dr Cian O’Leary from Pharmacy Department and Tissue Engineering and Research Group (TERG) headed by Prof Fergal O’Brien. Hanne grew 5 neuroblastoma cell lines on the 3 scaffolds: hard like a rock, soft and fluffy like a cotton wool and a jelly-like. All cells liked the jelly-like environment. This environment is similar to bone marrow – the most common site of neuroblastoma metastasis. We were excited to see the difference as it means we are one step closer to reconstruct this type of tumour spread.
John has expanded our exploration of our 3D neuroblastoma model by examining the content of exosomes – little parcels sent by cancer cells in 3D and as tumours grown in mice. We were thrilled to see a high similarity in the exosomal content. This finding additionally proved the great applicability of our 3D model as a tool to study neuroblastoma.
It is fantastic to see so knowledgeable and enthusiastic young researchers in my research group. This year, the team is multinational with the Irish students mixing with Belgian and Malaysian. All together they are cracking the code of neuroblastoma microenvironment and tumour cells communication through understanding main differences between conventional cancer cell models and tumours.
The big research plan of the entire team consists of more smaller and focused projects to be completed within 10-12 weeks. All projects are unrestricted, they are driven by the intellectual curiosity of these students. This way is full of ups and downs, frustrations and encouragements when techniques do not work or reagents do not come in as expected. Some cancer concepts can also work differently in the given settings. Simple questions are bringing more challenges than expected. But at the end of the road is the best reward – contribution to the conceptual advancement of neuroblastoma microenvironment.

This week can be rated for sure as feeling good, excited and accomplished. A UK based charity – Neuroblastoma UK has awarded a small grant to characterise a pre-clinical model of neuroblastoma which is a collaborative project between our lab and Tissue Engineering Research Group at RCSI. This project will study features of neuroblastoma cells growing on collagen-based scaffolds. The NBUK grant will contribute to one of the most expensive parts of the study – characterisation of cell secreting proteins using antibody-based profiling platforms.

Another research was accomplished yesterday – John Nolan had his Voice Viva examination and successfully defended his PhD Thesis. This 3 year PhD project was funded by the National Children’s Research Centre. As his supervisor, I am delighted for him and wish him best of luck in his research career.

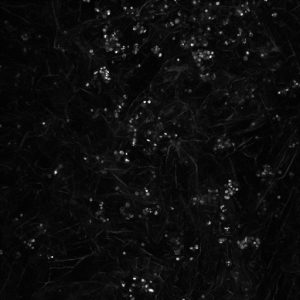
Continue research into 3D neuroblastoma models, we imaged cells growing on collagen based scaffolds using confocal microscopy. This technique is very popular in cell biology providing depth in cell imaging.
Here you can see cells growing on scaffolds: white dots – cells, irregular fibers – collagen containing scaffold.
The results are fascinating! Cell nucleus is in blue (DAPI), cell actin is in red (phalloidin). You will be able also to see how two cells ‘having a handshake’. It is happening just in the middle.

I am a medical student at Penang Medical College under a twinning programme with the Royal College of Surgeons in Ireland. I studied my pre-clinical years at RCSI Dublin. In the summer of 2015, I had the opportunity to join the RCSI Research Summer School (RSS) Programme. I was mentored by Dr Olga Piskareva, from Cancer Genetics, Molecular and Cellular Therapeutics (MCT) Department, RCSI. Being in this lab was simply one of the greatest experiences I have in my life; it was really rewarding.
My RSS project investigated the role of VDAC-1 protein on chemotherapy resistance in neuroblastoma. The only research focus of this lab is to find key players in neuroblastoma pathogenesis and to advance anti-cancer therapy.
I was entrusted with the task of splitting cells. I would plate them onto 96-well plates, add cisplatin drug and measure their viability afterwards. It may sound simple here, but the whole process required passion and hard-work.
Prior to this, I did not have any experience in the medical research field. During my first two weeks, everything seemed so tough; however, they became easier as the weeks flew by. My mentor, Olga, and the other staff and PhD students (Garret, John and Ross) were helpful and always guided me to explore my potentials. This programme taught me various new things which I would not have acquired on a normal day-to-day basis in school.

The people at Cancer Genetics were warm and wonderful. The hospitality, love and guidance cannot be quantified and words cannot express my immense gratitude towards them. It has been fascinating and I cherish every moment I spent there. We bonded over our weekly breakfast and tea sessions so well, and I am indeed grateful for being a part of this big family. It is my sincere wish that this positive spirit of togetherness will be preserved and will grow stronger in the future. This is something special, and I think ours is the best lab at RCSI!
Under this RSS, all the participants attended skills workshops and weekly Discovery Series lectures. We were also given a Biography of Cancer by Siddhartha Mukherjee to read; evidently a good read. Here are the links to the RCSI Research Summer School Student Testimonial Videos.
I returned to Penang Medical College to further my studies in my clinical years. I took part in the PMC Research Day 2016 in which I was awarded the First Prize in Oral Presentation. I would like to dedicate this success to Olga and everyone who has been with me throughout my time at Cancer Genetics. Without all the guidance, I would not have made it this far.
I strongly urge students to take part in the research opportunities, because you gain invaluable experiences that you do not get elsewhere. May whatever we do at the lab today make a difference in another person’s life someday in the future.
Mei Rin Liew
It seems I have got a conference season. Three conferences within 2 months – no complains though. This time I went to the Matrix Biology Ireland Meeting in Galway. It was fantastic mix of topics and speakers ranging from new approaches in bone and heart repair to new matrixes in reconstruction of body tissues and diseases in the lab to minimise use of animals in pre-clinical studies.
My talk was focused on neuroblastoma microenvironment and cell-to-cell communication through exosomes. I wrote about it in October post. I talked about things that did work and did not as well as new directions. One of the new directions is reconstructing neuroblastoma by growing neuroblastoma cells on collagen based scaffolds in 3D. Collagen constitutes most of our tissues to keep it shape and strength. These scaffolds are sponge-like matrixes built from collagen and other components. Of course cells grow differently on these matrixes. They have a different shape and growing properties in 3D. Neuroblatoma cells look like water drops on the cotton wool-like collagen scaffolds. In contrast, when they grow on plastic in 2D, they are flat. Studies show that cells in 3D respond to cytotoxic stress in a similar pattern as if being within a body (details in recent reviews 1-4). It would be a great breakthrough once these models are optimised for neuroblstoma research field. It will help to test all new and known drugs in the environment close to clinical settings. It could be a step forward to personilised therapies for children with neuroblastoma by isolating cancer cells, growing them in 3D and testing how they respond to all therapies available. It will facilitate more efficient design of treatment for relapsed or poorly responding tumours, sparing patients unnecessary rounds of chemotherapy and ultimately increasing survival.
I’ve always felt that a selection of abstracts for an oral presentation is biased. The overall background and views of conference organisers would affect works selected for an oral presentation. The same abstract was not selected for an oral presentation by one committee, but was supported by the other. Never give up!
Readings:
How we work with cancer cell lines?
The very first human cancer cell line was developed from a patient with an aggressive cervical cancer in 1951. This cell line was called HeLa after the patient name – Henrietta Lacks. This is the most popular and robust cancer cell line in biomedical research. Since then, other cancer cell lines were developed including neuroblastoma.
The first successful neuroblastoma ‘cell lines’ were cell populations from tumours that were adapted to grow for a short period in the lab environment in 1947. These tumour cell populations were used as a tool for diagnosis. This success inspired other researchers to develop long-term or immortal neuroblastoma cell lines. To date different neuroblastoma cell lines exist.
Cancer cell lines are sensitive and delicate in handling. They can only grow in the safe environment. Researchers have to protect them against bacteria, low temperatures, and too acidic/alkaline conditions. We protect cancer cells from bacteria contamination by handling them in cabinets where all plastic and media are sterile.

Cancer cells like to grow in conditions similar to conditions in human body. They like temperature of 36.6 – 37C. To achieve it special ‘green cell houses’ – CO2 incubators are built, which maintain the constant temperature, humidity and CO2 concentration.

The cell growth and well being are checked regularly using microscopes. Healthy cells are to have similar shape, even distribution and grow attached to the plastic surface. Most microscopes have a camera attached to the top and linked to a computer. It helps to take picture of growing cells and record changes in cell behaviour.

Recommended reading